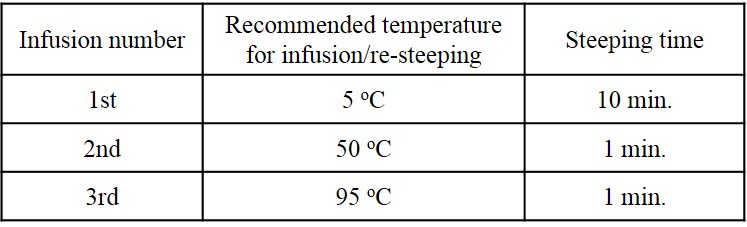
If a laser light is passed through some suspension which contains submicron or micron order particles, we can see the line of light scattering. For example, a water containing small amount of milk exhibits a red line of light scattering as follows.
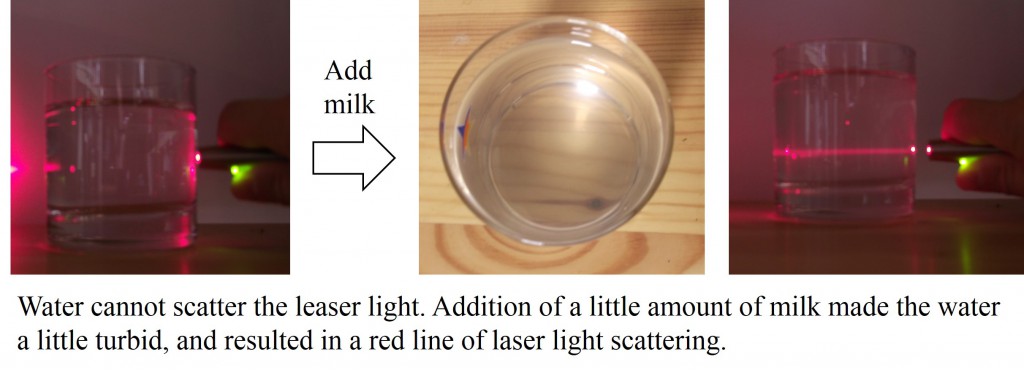
The scattered light indicates the information about size, shape and motion of particles suspended in the liquid. Thus, light scattering can be used to measure the size, size distribution and motion of submicron-sized particles [Mat099]. The technique are applied to not only chemical industry but also biological use [156], environmental measurement and food industry etc.
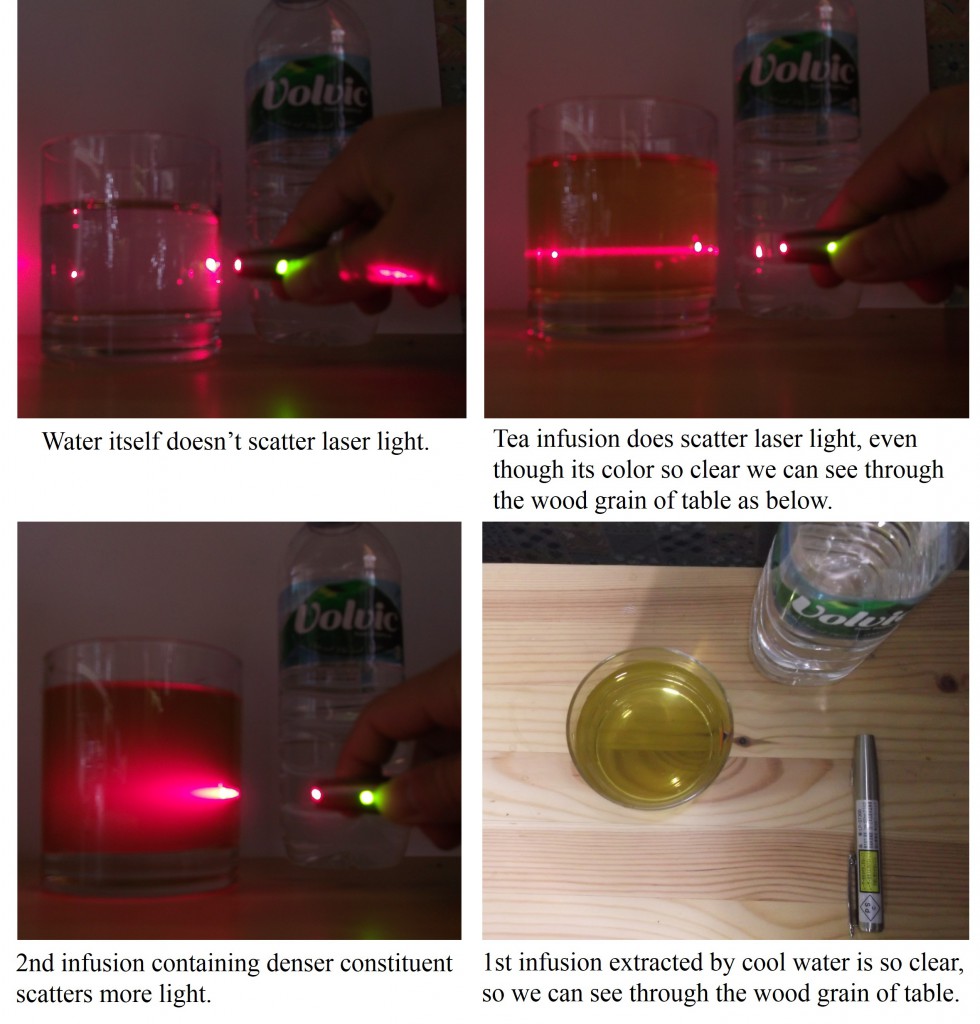
As mentioned and shown previously (Part 1), tea infusion can scatter the laser light, exhibiting the red line of light scattering. Compared with the other liquid having similar color, we can obviously find the difference in the property of light scattering as follows.

A liquid left side is an energy drink containing mainly amino acids and vitamins. Its yellow color is derived from vitamin B. Although the color of both liquid are so clear we can see through the wood grain of table, however, only tea infusion does scatter the laser light while energy drink doesn’t scatter.
It is supposed that the difference is caused by the existence of polyelectrolytes sized between micron and submicron order.
We can observe the red line of light scattering in the case of each tea such as black tea, oolong tea, pan-fired tea, and bottled green tea.

Even in the case of a bottled tea, which has been filtrated by porous media to remove or prevent sediments and sedimentation in its manufacturing process.
Based on the of Ito En Ltd.[P001], the components such as polysaccharides and proteins which cause sediments are removed by porous material filtration and silica adsorption. Beside, the patent article describe that D90 is more than 3500 micrometers, indicating that polyelectrolytes still remain in the bottled tea after filtration and adsorption process.
We can observe the aggregation process in a cup of tea, especially in the case of deep-steamed green tea as follows.

Aggregates grow bigger with elapse time, resulting in settling on the bottom of teacup.
This change affected the taste and mouthfeel of tea, I felt.
When I tasted the tea after infusion immediately, I could feel somehow viscous. After sedimentation, the supernatant was seemed be mild and have delicate sweetness, as same as before settling, while it seemed to have lower viscosity compared with before sedimentation. The sediments-rich part seemed to be more viscous and its taste itself seemed to be same as before sedimentation. On the other hand, I felt stronger aftertaste, which changed from slight astringency and green flavor to sweet taste.
This sense is the reason why I suppose polyelectrolytes and their aggregates are essential for the body, aftertaste, mouthfeel of teas.
In the next part, I’d like to show some images about my speculation.
<References>
[Mat099] Williams K. An education material for the biophysics in Yale University.(URL : http://keck.med.yale.edu/biophysics/talks/6Folta-Stogniew_tcm284-30259_tcm284-284-32.pdf)
[156] Peetermans,J.A. et al. (1987) : European Biophysics Journal 15:65-69.
[P001] Ito En Ltd. Bottled green tea drink and its manufacturing process. WO2012029131 A1 (2012.03,08)