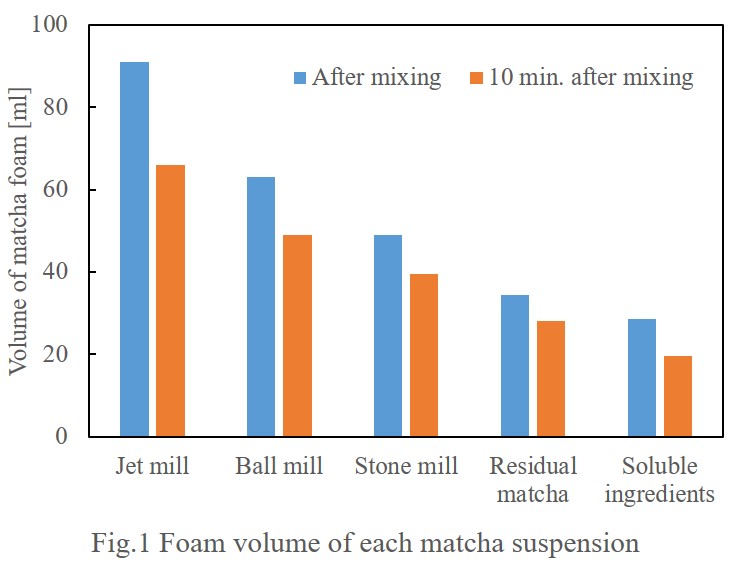
Speaking of foaming, those who learned chemistry may think some surfactants cause the bubbles. Indeed, tea leaves contains tea saponin, which can cause decrease of interface tension, resulting in foaming. However, Sawamura et al.(2012) clarified that not only water-soluble ingredients including tea saponin but matcha fine particles also contribute formation of matcha foaming. Fig.1 shows that the foam volume of the solution of matcha water-soluble ingredients is smaller than that of matcha after extraction of water-soluble ingredients.
The effect of particle size on foam formation was depicted as Fig.2.
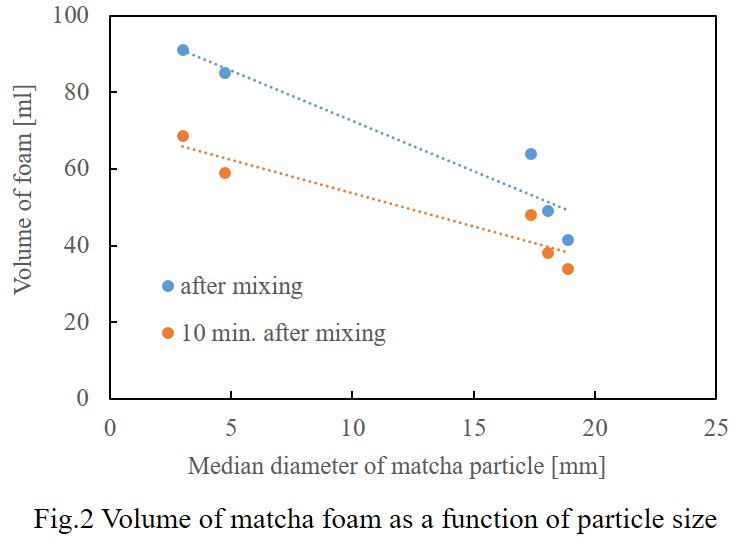
Smaller particles would contribute foam formation more.
Sawamura et al. speculated that the conjoining of matcha soluble ingredients and existence of matcha fine particles derive the unique foaming property of matcha.
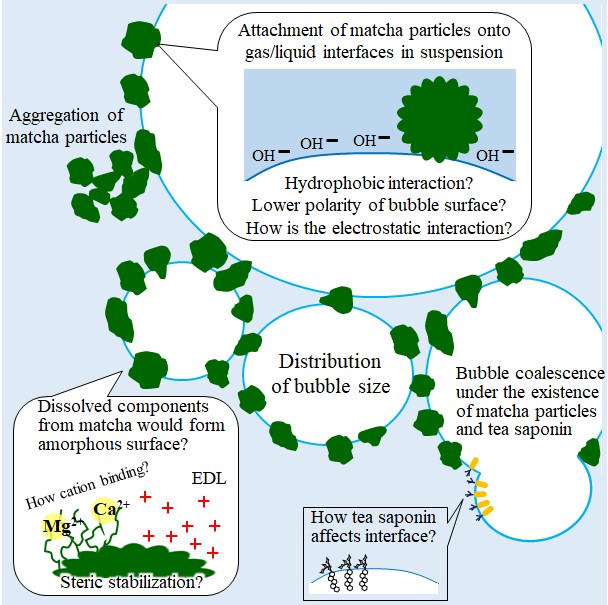
Instead of surfactants, fine particles can stabilize foams due to the tendency of fine particles to adsorb at the air-water interface.
According to Fujii & Murakami (2008)[414], foam stabilization with microparticles have been gaining concern again through over 100 years the first time Ramsden & Oxon found the phenomena[419].
Gonzenbach et al.(2006)[415] visualized the adsorption of fine particles onto bubble surface, causing ultrastable foaming. They also reported that the adsorption of short-chain amphiphiles onto suspended particles can derive hydrophobization of the surface, resulting in the higher stability of foams[413]. Re-adsorption of amphiphiles dissolved from matcha particles might contribute to the formation of stable foam.
In addition to interface active agents, it is speculated that the steric stabilization due to soft interface of matcha particles could be one of the contributions for foam stabilization. Fuji & Murakami (2008) reviewed that organic fine particles possessing polymer chains on their surface can stabilize bubbles in water due to the adsorption the particles onto air-water interface[415]. Interestingly, the organic hairy particles formed colloid crystal and keep the porous shape after absolute drying. Maybe colloidal crystal of matcha could be applied to develop new food products, for example absolute matcha meringue or matcha porous sheet etc.
<References>
[204] Sawamura et al. (2012) : Foaming Property and Foam Diameter of Matcha Varies with Particle Size, Nippon Shokuhin Kagaku Kaishi, 59:109-114. (in Japanese, English abstract is available.)
[413] Gonzenbach, U.T. et al.(2006) : Stabilization of Foams with Inrganic Colloidal Particles, Langmuir 22:10983-10988.
[414] Fuji & Murakami (2008) : Foams Stabilized with Microparticles, Surface Technology, 59:33-38. (in Japanese, English abstract is available.)
[415] Gonzenbach, U.T. et al.(2006) : Ultrastable Particle-Stabilized Foams, Angewandte Chemie International Edition, 45:3526-3530.
[419] Ramsden & Oxon (1904) : Separation of solids in the surface-layers of solutions and ‘suspensions’ (observations on surface-membranes, bubbles, emulsions, and mechanical coagulation). – Preliminary account, Proc. R. Soc. Lond. 72:156-164.
